|
||||||
|
||||||
| Home Nation World Business Opinion Lifestyle China Focus ChinAfrica Video Multimedia Columnists Documents Special Reports |
|
||||||
|
||||||
| Home Nation World Business Opinion Lifestyle China Focus ChinAfrica Video Multimedia Columnists Documents Special Reports |
| Nation |
| The Healing Touch |
| Program to make new medicines domestically results in easier, cheaper treatment |
| By Wang Hairong · 2020-01-16 · Source: NO.4 JANUARY 23, 2020 |
 Medical Breakthrough Geng Meiyu (left), key inventor of the drug GV-971,interacts with her colleague at the Green Valley Institute in Shanghai on November 3. The home-grown drug for treating Alzheimer's disease has been approved by the National Medical Products Administration to hit the market after research efforts of 22 years (XINHUA)
When Siyu, then an undergraduate student in east China, went to study in the U.S. two years ago, she had several usual items on her agenda: taking courses, learning the local culture, making friends and sightseeing. However, there was one item that was unusual for a student—getting human papillomavirus (HPV) vaccine shots. At that time, China didn't make the vaccines, which are used to protect against the HPV that can lead to cervical cancer in women. When the imported vaccines first entered the Chinese mainland market, the supply could not keep up with the surging demand and people had to make reservations well in advance. According to the National Cancer Center in 2015, six out of 100 Chinese women diagnosed with cancer had cervical cancer. "I heard the chance to get the vaccine was as slim as drawing lots," Siyu, who didn't want to give her full name for privacy, told Beijing Review. Several close friends of hers had traveled to places like Hong Kong to get the shots. So she decided to get vaccinated while studying in the U.S. However, things have changed since then. On December 31, 2019, China's drug regulator announced it had approved Cecolin, the first home-made HPV vaccine. Siyu is glad that women like her can now get vaccine protection against the virus easier and cheaper. The Chinese vaccine was developed by INNOVAX, a biotechnology company based in Xiamen, Fujian Province in southeast China, in cooperation with Xiamen University. According to the National Medical Products Administration (NMPA), clinical trials showed that it protected 97.8 percent of the recipients against persistent infection by HPV strains 16 and 18. INNOVAX has become the third company in the world to produce an HPV vaccine, in addition to UK-based GlaxoSmithKline and U.S.-based Merck & Co. The company said it has also developed a nine-valent HPV vaccine that can protect recipients against nine types of HPV and is currently under clinical trial. 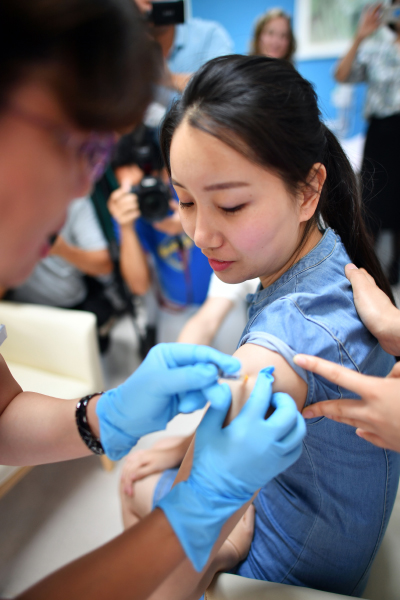 A woman receives an injection of an imported nine-valent HPV vaccine at a hospital in Beijing on May 30, 2018 (XINHUA)
New drugs bonanza While many people are excited about the new vaccine, they might not be aware that a government initiative supported its development—the national science and technology major projects for new drug development, popularly known as the new drugs program. Launched in 2008, it is one of the 16 major programs listed in the outline of the national long- and medium-term development plan for science and technology (2006-20), alongside the development of large aircraft, manned space flights and lunar probes. Chinese researchers had developed 139 new certified medicines with support from the program, Liu Dengfeng, Commissioner of the National Health Commission's Department of Health Science, Technology and Education, said at a press conference in July 2019. Among these new medicines, 44 are Class I drugs that had not been marketed in China or overseas before, according to Liu, who is also deputy head of the office managing the program. Fourteen of these new drugs, developed in the recent two years or so, are mainly for the treatment of malignant tumors, AIDS and skin diseases, Chen Kaixian, deputy chief technical engineer of the program, said. Benefits of innovation The ultimate goal behind developing new drugs is to see people benefit from science and technology innovation, Liu said. The program sponsors the development of urgently needed drugs for treating serious diseases and has improved patients' access to safe and effective medicines, he added. On November 2, 2019, the NMPA granted conditional approval to Oligomannate, or GV-971, a drug derived from seaweed to treat Alzheimer's disease. It was developed by Chinese pharmaceutical company Green Valley, with some funds from the new drugs program. According to the NMPA, clinical trials showed that the drug can improve the cognitive function of patients suffering from mild Alzheimer's disease and prevent its progression. The approval of the new drug is a breakthrough since in the previous 17 years, no new drug had been approved in the world to treat Alzheimer's disease, the NMPA stated. Alzheimer's Disease International, the global organization of Alzheimer associations, estimated that 46.8 million people worldwide lived with dementia in 2015, including 10 million in China. Its global total incidence is expected to almost double every 20 years. Another notable achievement under the sponsorship of the program is a recombinant Ebola vaccine, which was approved by China Food and Drug Administration, the NMPA's predecessor, in October 2017. The vaccine was independently developed by Chinese researchers, making China the third country after the U.S. and Russia to produce an Ebola vaccine. The Chinese vaccine is available in the form of powder. Compared with the liquid vaccines produced in the other two countries, it is more stable and more convenient to transport. Ebola is classified by the World Health Organization (WHO) as one of the most serious diseases threatening humans. The Ebola epidemic in West Africa in 2014-16 claimed at least 11,310 lives, according to a WHO situation report in June 2016. In addition to improving access to drugs, the program has also made drugs more affordable, Chen said. He gave the example of a foreign drug used for treating hepatitis C. It costs $1,000 per tablet and the whole course of treatment costs more than $80,000. But a similar medicine developed in China has lowered the expenses of the entire course of treatment to no more than $10,000. The cost will be halved in the near future, Chen said. According to Liu, many new drugs developed under the program's sponsorship have been included in the catalog of drugs covered by China's basic health insurance.  Chen Wei (center, back row), the Chinese researcher who led the development of an Ebola vaccine, visits children orphaned by the disease during clinical trials of the vaccine in Sierra Leone on November 8, 2015 (XINHUA)
The drugs developed by Chinese researchers also offer more choices to patients in other countries. Liu said as of the end of 2018, more than 280 China-developed generic medicines had been registered in Europe and the U.S., while four vaccines and 23 chemical preparations had been pre-approved by WHO. He added that currently, more than 100 China-developed new drugs supported by the program are under clinical trial in Europe and the U.S. One of these, Brukinsa (Zanubrutinib), was granted accelerated approval by the U.S. Food and Drug Administration on November 14, 2019. It is used to treat adult patients with mantle cell lymphoma—white blood cell cancer—provided they have received at least one prior therapy. The drug was developed by BeiGene, a biopharmaceutical company founded in Beijing in 2010 to produce anti-cancer drugs. The company opened its first U.S. office in the Boston area in 2015, and went public on Nasdaq in 2016. With the support of the new drugs program, the internationalization of traditional Chinese medicine (TCM) has also made remarkable progress, Liu said. Five TCM drugs have been approved in Canada as traditional medicines, and 17 are under clinical trial or in the process of getting approval in foreign countries. Copyedited by Sudeshna Sarkar Comments to wanghairong@bjreview.com |
About Us | Contact Us | Advertise with Us | Subscribe
|
||
| Copyright Beijing Review All rights reserved 京ICP备08005356号 京公网安备110102005860号 |